Metals
- Metals in their a pure state have lustre. (metallic sign), are ductile ( that can be drawn into thin wire) and malleable ( that can be hamered into thin sheets). Metals conduct heat and electricity.
- EXCEPTIONS: All Metals except mercury are solid at room temperature. All Metals except gallium and caesium have high melting points. Gallium and Caesium melts on palm. All Metals except Alkali Metals (Lithium, sodium and potassium) are hard. But Alkali Metals can be cut through knife. Alkali Metals have low melting points and low densities.
- Metal react with oxygen to form basic oxide.
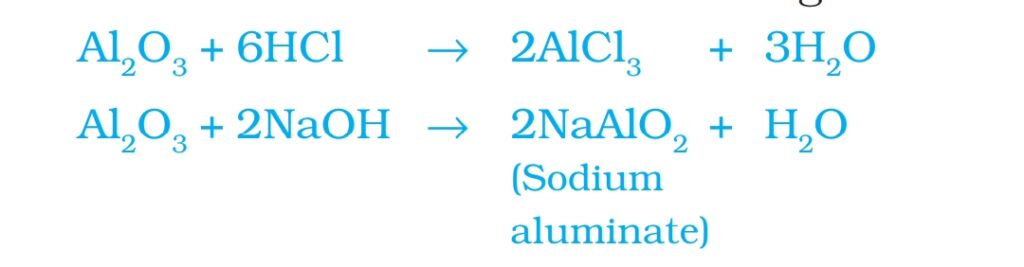
- Aluminium and Zinc oxides show both acidic and basic behavior. ZnO and Al2O3 react with acids as well base. These oxides are called amphoteric oxides.
Non-Metals
- Non- Metals not show the properties like ductility, malleability, sorority etc. They do not conduct heat and electricity.
- EXCEPTIONS: Iodine is a lustrous non metal. AttrGraphite a form of carbon conduct electricity. Different forms of carbon like diamond and graphite are called allotrope. Diamond have very high melting and Boling points and graphite conducts electricity.
- Non metal oxides are acidic in nature.
- Alkali: alkali are the metal oxide that can completely dissolve in water. Example NaOH ( caustic soda) and KOH ( caustic potas).
Reaction of Metals with Water

Reaction of metal with Acid
- Metal react with Acid to produce metal salt and hydrogen gas. But could not produce hydrogen gas when metal react with nitric Acid (HNO3). This is due to the fact that nitric Acid is strong oxidizing agent, it oxidised hydrogen gas into water and itself reduced to oxide of nitrogen. But Mg and Mn react with very dilute nitric Acid to produce H2 gas.
Define reactivity series.
The reactivity series is a list of Metals arranged in the order of their decreasing activities.

Why hydrogen placed in the reactivity series of Metals?
Ans. Hydrogen has intermediate position in the reactivity series . Metals above Hydrogen are considered moderately or highly reactive Metals and Metals below Hydrogen are considered low reactive Metals.
Ionic compounds
Compounds form by transfer of electron are called ionic compounds. Elements which can donate electrons are called cations and the Elements which can accept electrons are called anions.
- NaCl
- Na2O
- Al2O3
Ans.
Write the properties of ionic compounds/electrovalent compounds.
- Ionic compounds are solid and hard due to strong force of attraction between cations and anions. These compounds are brittle.
- High melting and boiling points as considerable amount of energy is required to break the inter-ionic attraction.
- soluble in water but insoluble in organic solvents like kerosene, petrol etc.
- conduct electricity in molten form as ions are not free to move in solid state.
Define the following terms:
- Minerals
- ores
- Gangue
Ans. 1. Minerals: compounds of Metals/non metals found in earth crust are called minerals.
2. Ore: Those minerals in which appreciable percent of metal are present. Metals can be extracted profitably from ore. All ores are minerals and all minerals are not ores.
3. Gangue: impurities found with the ores are called gangue. These may include dust, soil and other undesirable compounds.
Q. Define concentration process.
Ans. The process of removing of gangue from ore is called concentration process. It is also called enrichment of ore.
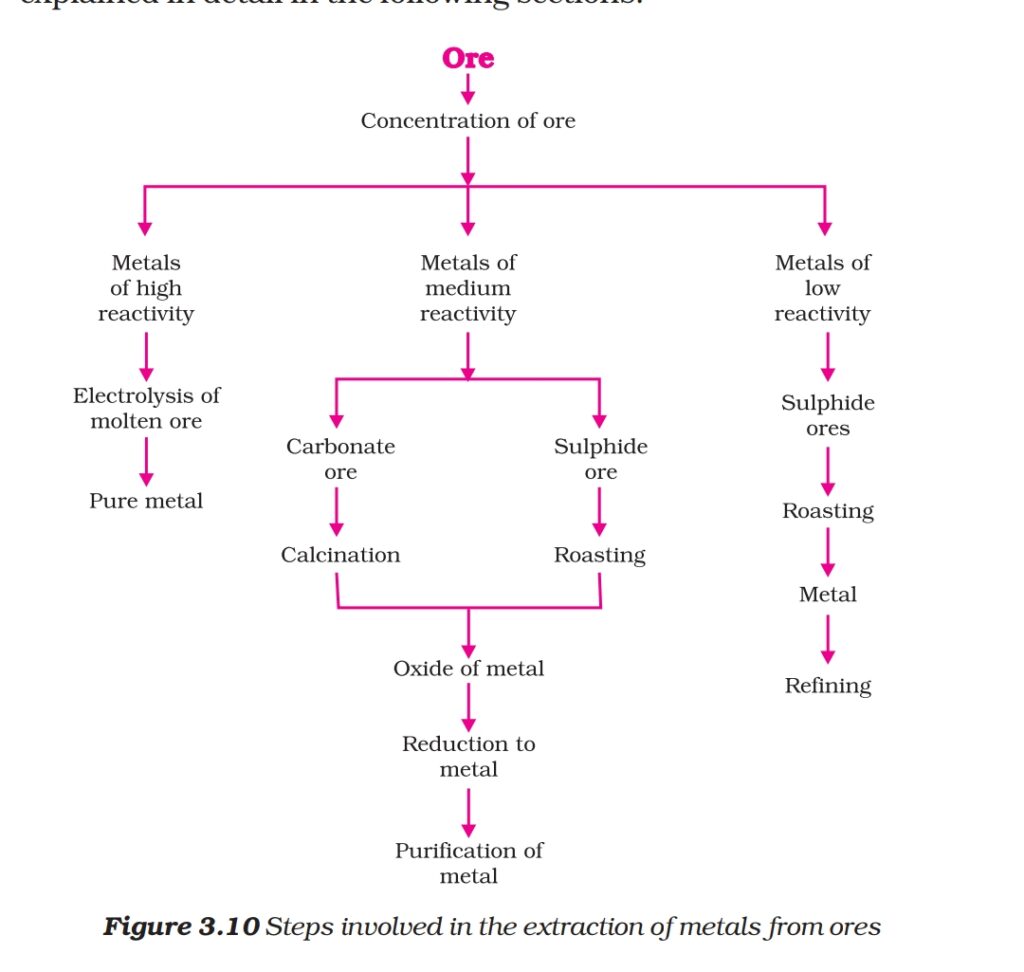
Q. Draw a flowchart which which list the processes of obtaining Metals from their ore.
Q. Define:
- Roasting
- calcination
- reduction of metal oxides
Ans. 1. Roasting: Heating strongly sulphide ore in excess air to obtain metal oxide is called Roasting.

2. Calcination: Heating strongly carbonate ore in limited air to obtain metal oxide is called Calcination.

3. Reduction of metal oxide: it is a method of obtaining metal from metal oxide.
Q.) How Metals of low reactivity obtained from their ores?
Extraction of Hg from HgS( cinnabar):
Roasting of HgS: Heating HgS with excess air to obtain HgO

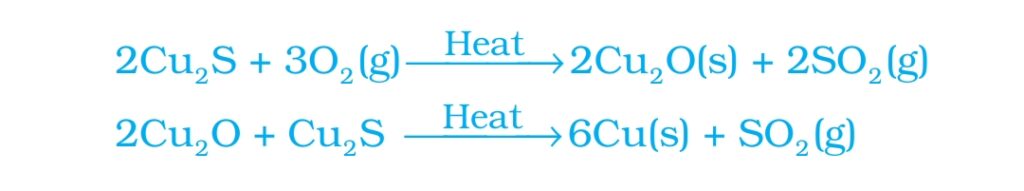
Extraction of Cu from Cu2S
Step1-Roasting of Cu2S: Heating Cu2S in excess air to get Cu2O
Step2- reaction of Cu2O with Cu2S to obtain Cu
Heating HgO to get Hg:
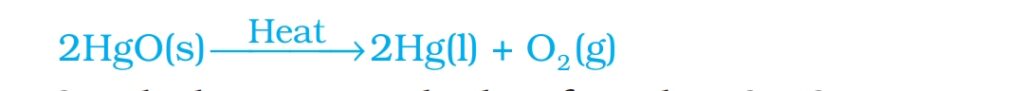
Q. How metal in middle in the reactivity series obtained from their ore?
Ans. Metal like zinc can be obtained from their sulphide ore ore and carbonate ore by following ways.
obtaining Zn from ZnS
- Roasting of ZnS: Heating strongly ZnS in excess air to obtain ZnO.
obtaining Zn from ZnCO3
- Calcification of ZnCO3: Heating strongly in limited air to get ZnO


Step 2: obtaining Zn from ZnO by reduction with hot coke (carbon).
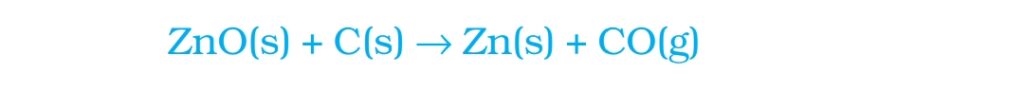
Q.) How Metals on top of reactivity series obtained from their ores?
Ans. Highly reactive Metals obtained by electrolysis of their molten ore. eg Na, Mg,Ca, Al
for example electrolysis of NaCl to get Na following reactions takes place at anode and cathode.
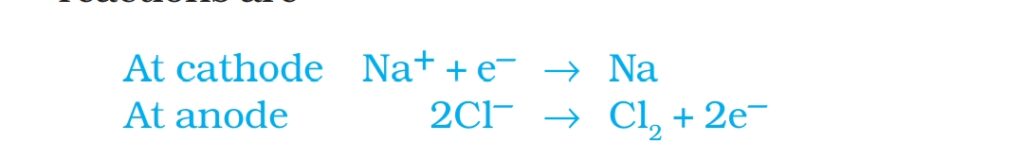
Note: displacement reaction with highly reactive Metals like Na, Ca, Al are used as reducing agents.
example. When manganese dioxide is heated with aluminum powder following reaction takes place.
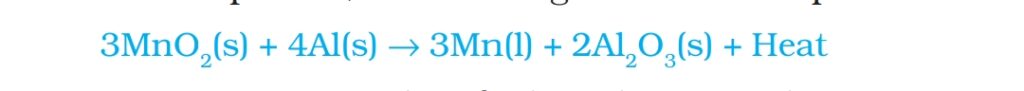
Thermite Reaction: reaction of Aluminium with ferric oxide is highly exothermic. Iron obtained in molten state is used fill cracks in railway track is called Thermite reaction.
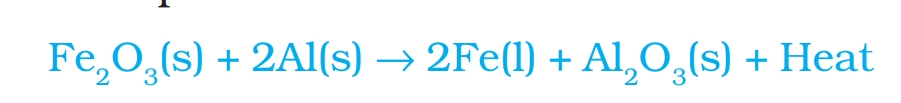
Q.) Explain refining of metal?
Ans. After extraction of metal from the ore , the purity of metal obtained is not 100%. Some impurities mixed with the metal. These impurities can be removed by the process of electrolysis called electrolytic refining.
Electrolytic refining of copper : Impure copper is made anode pure copper is made cathode. Electrolyte can be copper sulphate. When electricity is passed through the solution, impure copper dissolve in the the solution and same amount of pure copper from solution deposited on cathode. Impurities are collected near anode called anode mud.

Q. Define corrosion and give some practical examples.
Ans. When Metals expose to moisture and air they start combining with these and their strength and properties are called corrosion.
some examples of corrosion are:

Q. Define alloy? Give examples.
Ans. Alloy is homogeneous mixture of two or more metal and a non metal to improve quality. There is very less corrosion found in alloys.
Examples:
| Steel | iron , carbon and other metals |
| Stainless steel | iron , nickel, chromium |
| Amalgam | mercury + any other metals |
| Brass | Copper + Zinc |
| Bronze | copper + tin |
| Solder | lead + tin |
Important Notes:
- 0.05% carbon is added to iron to make it hard.
- the electrical conductivity and melting points of an alloy is less than that of pure metal.
- 24 carat got is very soft. 22 carat gold is used in India that means 22 parts of pure gold and 2 parts of silver or copper.
- aqua Regia is mixture of HCl and HNO3 in the ratio of 3:1. It can dissolve gold and platinum.
- region behind non corrosion of iron pillar of Delhi- formation of a layer of magnetic oxide ( Fe3O4) on its surface.
- electrolytes are compounds that can dissociate into ions in aqueous solution.
- Galvanisation is method of depositing a layer of Zinc on chipper Metals by the process of electrolysis.
- Aluminium is very reactive metal but it is used in making utensils as a thin layer of aluminium oxide is formed on its surface which prevent corrosion.
Introduction to Metals and Non-Metals
Metals and non-metals represent two fundamental categories in the study of chemistry. Understanding these elements is not only crucial for academic examinations but also for practical purposes in everyday life. The properties, uses, and distinctions between metals and non-metals form a foundational aspect of material science.
Importance in Academic Examinations
The chapter on metals and non-metals is significant in various examination syllabuses, particularly at the high school and college levels. Students are often tested on the characteristics, reactions, and applications of both types of elements. Mastery of this subject matter can greatly influence a student’s overall performance in chemistry. Therefore, comprehending the differences and similarities between metals and non-metals is imperative for achieving academic success.
Practical Applications of Metals and Non-Metals
Beyond academic evaluation, the understanding of metals and non-metals plays a vital role in practical applications across multiple industries. Metals like iron, aluminum, and copper are widely used in construction, electrical wiring, and manufacturing. Conversely, non-metals such as sulfur and phosphorus find their applications in fertilizers and explosives. This knowledge not only pertains to theoretical concepts but also impacts decision-making in industrial practices and technological advancements.
…..Published by Rahman Sir.