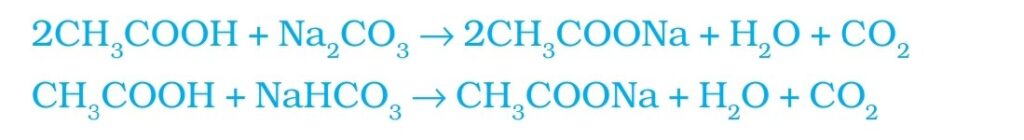Important Definitions
Hydrocarbon: Compounds of carbon and hydrogen is called hydrocarbons. These compounds are also called organic compounds. eg CH4, C2H4.
| Covalent bond | Ionic Bond |
| Bond formed by sharing of electrons between atoms. Compounds so formed are called covalent Compounds. Covalent compounds have low melting and boiling points due to low intermolecular force of attraction. eg. Bonding in CH4 | Bond formed by transfer of electrons. Compounds so formed are are called ionic compounds. High melting and boiling points due to high inter ionic attraction. eg. Bonding in NaCl |
Reason behind covalent/sharing nature of Carbon.
- It could gain four electrons forming C4– anion. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
- It could lose four electrons forming C4+ cation. But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Reason for Versatile nature of carbon , large number of carbon compounds.
- Catenation property: Carbon has the unique ability to form bonds with other atoms ofcarbon, giving rise to large molecules. This property is called catenation.
- compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings.
- carbon atoms may be linked by single, double or triple bonds. Compounds of carbon, which are linked by only single bonds between the carbon atoms are called saturated compounds.
- Tetravalency: Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other mono-valent element.
- bonds that carbon forms with most other elements are very strong making these compounds exceptionally stable. i.e carbon-carbon bond is very strong and hence stable. This gives us the large number of compounds.
Catenation property of Silicon
Silicon forms compounds with hydrogen which have chains of upto seven or eight atoms, but these compounds are very reactive.
Compare Saturated and unsaturated hydrocarbons
| Saturated Hydrocarbons | Unsaturated Hydrocarbons |
| compounds having only single bond are called saturated hydrocarbon. eg Alkane, cyclo alkane. These compounds give clean blue flame while burning. | Compounds of carbon having double or triple bond are called unsaturated hydrocarbon. eg Alkene, Alkyne, benzene. Unsaturated carbon compounds will give a yellow flame with lots of black smoke. |
Q. Give a test to distinguish saturated and unsaturated hydrocarbon.
Ans. Bromine water test: Bromine water decolorizes when it reacts with alkenes or alkynes, which have unsaturated carbon-carbon bonds. Bromine water doesn’t react with alkanes, which have single bonds, so the color of the bromine water doesn’t change.
Q. Define:
- Homologous series
- Functional Group
- Isomerism
Ans. 1. Homologous Series: Compounds of carbon which have same functional group but differ by CH2 in molecular formula and 14u in molecular mass. eg CH4, C2H6,C3H8
2. Functional group: Hetroatoms or group of atoms which when attached to a carbon chain (Alkyle-R) dominates the property of the compounds are called Functional group. Some Functional group are given below.
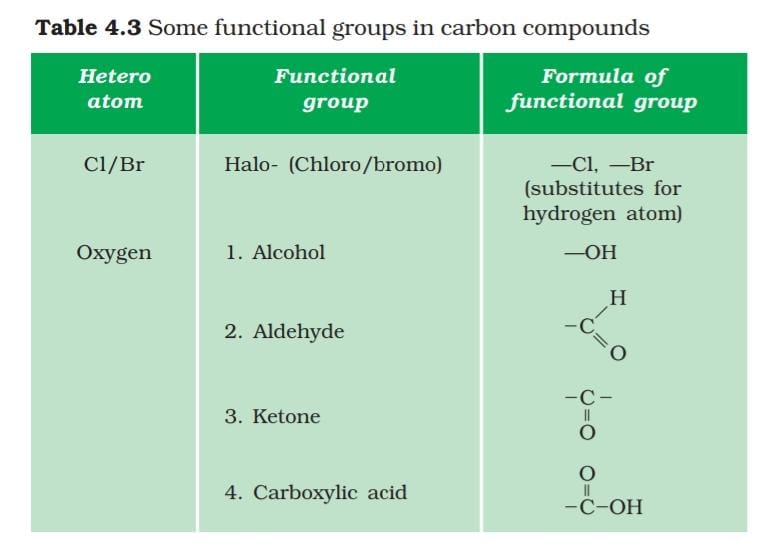
3. Isomerism: Compounds which have same molecular formula but different structures are called Isomerism and the phenomena is called Isomerism.

- Cyclo alkane is isomers of alkene
- aldehyde and ketone are isomers with molecular formula CnH2n-O
Nomenclature/naming
| Number of carbon | Prefix |
| 1 | Meth |
| 2 | Eth |
| 3 | prop |
| 4 | but |
| 5 | pent |
| 6 | hex |
| 7 | hept |
| 8 | oct |
| 9 | non |
| 10 | dec |
| 11 | undec |
IUPAC NOMENCLATURE
- Alkane. Prefix+ane example CH4 is meth+ane i.e methane (suffix is ane)
- Alkene. Prefix+ene example C2H4 is eth+ene i.e ethene (suffix is ene)
- Alkyne. Prefix+yne example C3H4 prop+yne i.e propyne. (Suffix is yne).
- Alcohol: Alkan+ol ( e from alkane is eliminated ) i.e Alkanol example CH3OH is methan+ol=Methanol.
- Aldehyde: Alkan+al i.e Alakanal example CH3CHO is ethan+al=ethanal (count total number of carbon)
- Ketone: Alkan+one i.e Alkanone example CH3COCH3 is propan+one=propanoic.
- Carboxylic Acid: Alkan+oic acid example CH3COOH is ethan+oic acid
- Ester: Alkyl Alkanoate example CH3COOCH3 is methyl Ethanoate (smaller carbon chain -alkyl).
General formulas
- Alkane: CnH2n+2 example CH4, C2H6, C3H8
- Alkene: CnH2n example C2H4, C3H6, C4H8
- Alkyne: CnH2n-2 example C2H2, C3H4, C4H6
- Alkyl Group: Represented by “R”. General formula is C2H2n+1-. Any functional group attached to alkyl. It is known as carbon chain. Examples R-OH is alcohol, R-CHO is aldehyde, R-CO-R’ is ketone, R-COOH is carboxylic acid, R-COO-R’ is ester and R-X is halo alkane where X may be F, Cl, Br, I.
- Alcohol: CnH2n+2-O example C3H8O= C3H7OH is propanol
- Aldehyde: CnH2n-O example C3H6O= C2H5CHO is propanal
- Ketone: CnH2n-O example C3H6O= CH3COCH3 is propanone.
- Carboxylic Acid: CnH2n-O2 example C2H4O2=CH3COOH is ethanoic acid.
Q. Write two different cyclic compounds with carbon number 6 in which one is saturated and another is unsaturated.
Ans. Cyclo hexane is saturated where benzene is unsaturated.
Q. Define allotropes. Compare properties of graphite and diamond.
Ans. Different form of same element which differ in physical properties due to Different structures. Examples diamond, graphite , Buckminsterfullerene (C-60).
| Graphite | Diamond |
| one carbon atom is bonded with 3 other carbon atoms | one carbon atom is bonded with 4 other carbon atoms |
| unsaturated as one of 3 bonds is double bond | saturated as all bonds are single |
| Shape is Hexagon | Shape is tetrahedral |
| soft and slippery | rigid |
| good conductor of electricity | poor conductor of electricity |
Q. Write chemical properties of hydrocarbons.
Ans. Chemical properties of hydrocarbon can be broadly categorized into 4 parts.
- Combustion
- oxidation
- Addition or hydrogenation reaction
- Substitution reaction
Combustion: hydrocarbon burn in the presence of oxygen to produce carbon dioxide, water, heat and light. Saturated hydrocarbon give clean blue flame while unsaturated hydrocarbon give yellowish flame with lot of smoke.
CH4+O2———->CO2+H2O+ heat and light
C2H5OH+O2———–>CO2+H2O+ heat and light
Oxidation: Ethanol is oxidized into ethanoic acid in the presence of alkaline KMnO4 ( potassium permanganate) or acidified K2Cr2O7 (potassium dichromate).
C2H5OH……Alkaline KMnO4 or Acidified K2Cr2O7—->CH3COOH
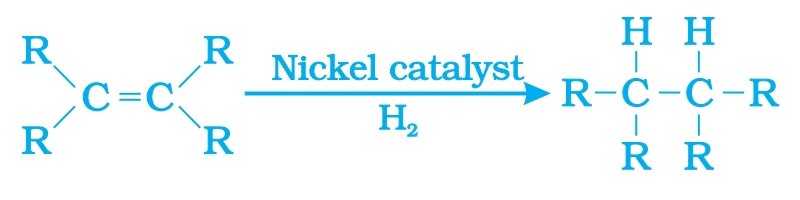
Addition reaction: Unsaturated hydrocarbon like alkene react with hydrogen gas in the presence of Nickel or Palladium catalyst to give saturated hydrocarbon like alkane. Vegetable oil which is an unsaturated hydrocarbon is converted into Vegetable ghee by the process of hydrogenation.
Substitution reaction: Saturated hydrocarbon like alkane react with chlorine in the presence of sun light to form alkyl halide (halo alkane ) and hydrochloric acid.
CH4+Cl2—-sun light—->CH3Cl+HCl
CH3Cl+Cl2——–sun light——>CH2Cl2+HCl
CH2Cl2+Cl2——sun light——–>CHCl3+HCl
CHCl3+Cl2——-sun light———>CCl4+HCl
CH3Cl is chloro methane. CH2Cl2 is dichloro methane. CHCl3 is tricolor methane or chloroform and CCl4 is carbon tetrachloride.
Q. Define:
- Soap
- Detergent
- Miscelle
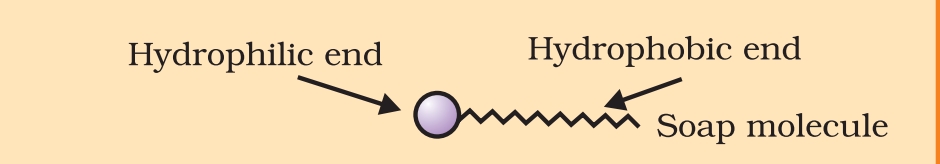
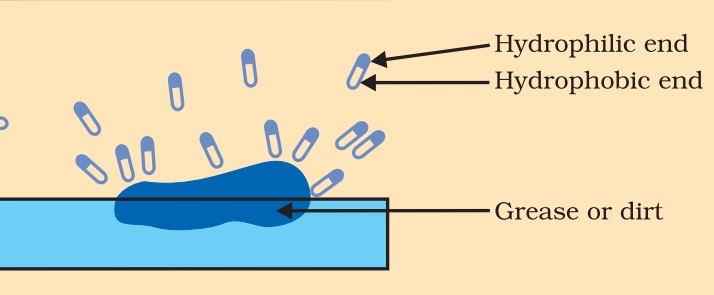
Ans. 1. Soap: Soap are sodium or potassium salt of long chain carboxylic acid. It has two ends, one is carbon chain end called hydrophobic end of saop and another is ionic end Called hydrophilic end of Soap. Soap are not effective in hard water as ionic end of soap reacts with calcium and magnesium ions present in hard water to form insoluble precipitate called scum.
2. Detergent: Detergents are generally sodium salts of sulphonic acids or ammonium salts with chlorides or bromides ions. Charged ends of detergents do not form insoluble precipitates with the calcium and magnesium ions in hard water. Thus, they remain effective in hard water. Detergents are usually used to makeshampoos and products for cleaning clothes.
3. Miscelle: In water, the soap molecule is uniquely oriented which helps to keep the hydrocarbon part outside the water. When the clusters of molecules are formed then hydrophobic tail comes at the interior of the cluster and the ionic end comes at the surface of the cluster and this formation is called a micelle.
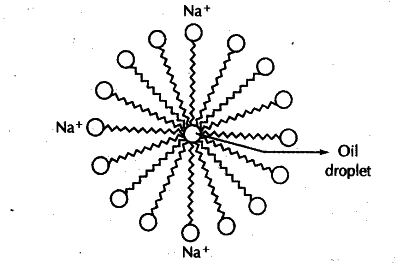
Q. Explain the working of soap?
Ans. When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. Thus, clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away.

Q. List physical properties of ethanol/alcohol.
Ans.
- Ethanol is a liquid at room temperature. It’s melting point is 156K and boiling point is 351K.
- It is active ingredient of all alcoholic drinks.
- Because it is agood solvent, it is also used in medicines such as tincture iodine, coughsyrups, and many tonics.
- Ethanol is also soluble in water in all proportions.
- Consumption of small quantities of dilute ethanol causes drunkenness.
- Intake of even a small quantity of pure ethanol (called absolute alcohol) can be lethal. Also, long-termconsumption of alcohol leads to many health problems.
Q. Write physical properties of ethanoic acid/acetic acid.
Ans.
- Belongs to a group of acids called carboxylic acids.
- 5-8% solution of acetic acid in water is called vinegar and is used widely as a preservative in pickles.
- The melting point of pure ethanoic acidis 290 K and hence it often freezes during winter in cold climates. This gave rise to its name glacial acetic acid.
- Unlike mineral acids like HCl, which are completely ionised, carboxylic acids are weak acids.
Q. Write chemical properties of ethanol.
Ans. Following are the chemical properties of ethanol:
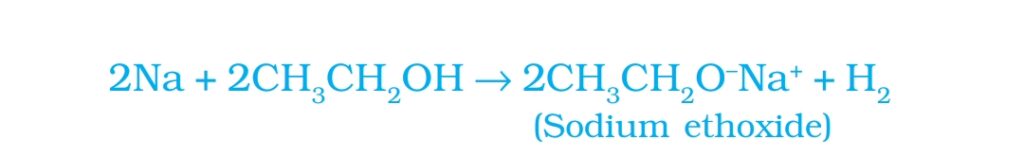
- Reaction with sodium: Sodium react with ethanol to form sodium ethoxide and hydrogen gas.
- Conversion of ethane to ethene: Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene. Conc. Sulphuric acid acts as dehydrating agent.

Q. List chemical properties of ethanoic acid.
Ans.
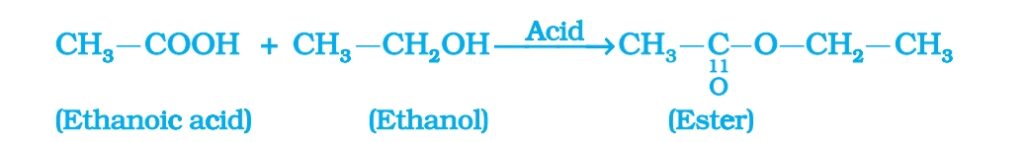
- Esterification reaction: Ethanoic acid react with absolute ethanol in the presence of an acid catalyst to form ester. Esters are sweet smelling substances. Easters are used in making perfumes and flavouring agents.
2. Saponification Reaction: Esters react in the presence of an acid or base to give alcohol and carboxylic acids. It is used in the preparation of soap. So this Reaction is called Saponification Reaction.
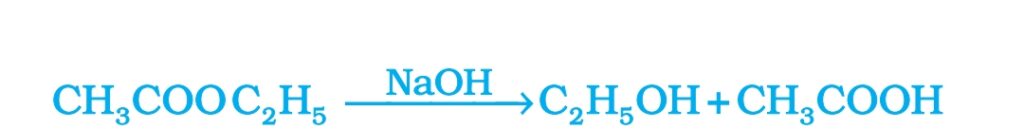
3. Reaction with base: Ethanoic acid react with base such as NaOH to form sodium acetate and water.

4. Reaction with carbonate and hydrogen carbonate: Ethanoic acid react carbonate and hydrogen carbonate to form sodium acetate, water and carbon dioxide. This reaction is used for testing ethanoic acid . CO2 formed extinguish a burning candle or make lime water milky.